Vanessa Lamers is the Assistant Director of Performance Management and Quality Improvement at PHF.
To support health departments, hospitals, healthcare organizations, and governments in the roll-out of the vaccine, the Public Health Foundation (PHF) has developed this overview of the Pfizer-BioNTech COVID-19 Vaccine logistics. Complete information with additional details can be found on Pfizer’s website: https://www.cvdvaccine-us.com/. This information was updated on February 8th, 2021.
This past weekend, the first vials of Pfizer vaccines shipped from the Midwest and headed to the arms of hospital workers and
healthcare professionals around the world. Results from clinical trials indicate the Pfizer vaccine is 95% effective with two-doses, providing a promising pathway out of the COVID-19 pandemic. However, the preservative-free vaccine must be transported and stored at ultra-cold temperatures, posing a challenge for facilities receiving the vaccine.
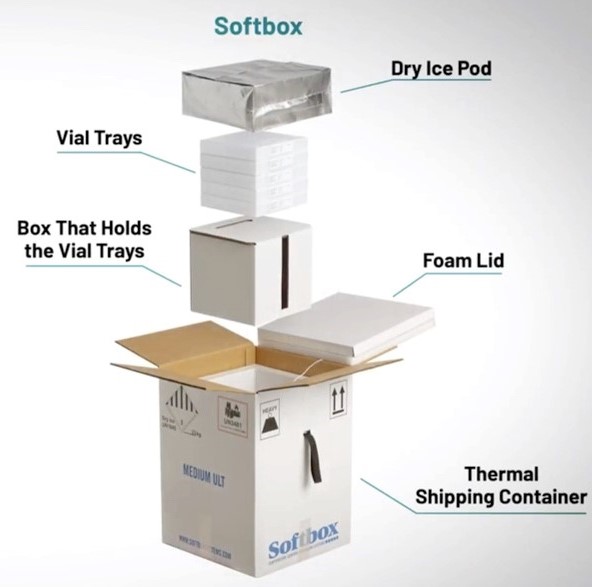
To ensure ultra-cold temperatures, the vaccine vials are
shipped in a thermal shipping container containing dry ice and continuously monitored with a temperature monitoring device on the foam lid.
The box appears medium-sized, but can
weigh up to 80 pounds, as dry ice is twice as heavy as regular ice. Facilities receiving the package may want to be prepared with a dolly or forklift, depending on how many boxes they are receiving. The Public Health Foundation developed a
dry ice safety fact sheet for the Centers for Disease Control and Prevention (CDC) to help with safe handling and storage of dry ice.
The temperature monitor on the foam lid keeps track of temperature fluctuations and provides email alerts and reports. Upon opening, the temperature monitor will flash green to indicate the box stayed within range during transport or will flash red if there was a problem that needs to be investigated.

Upon arrival, the
thermal shipping box should be stored and opened in a well-ventilated area. Dry ice is carbon dioxide in solid form and goes from a solid to a gas (sublimates) as it warms. In a closed space, carbon dioxide can replace oxygen and cause suffocation.
Waterproof, insulated gloves must be worn when handling dry ice, along with good eye protection.
The best storage for these ultra-cold vaccines is an ultra-cold freezer. However, Pfizer has provided additional specifications and details on storage depending on the type of site or “archetype” (e.g., permanent, redistribution, mobile, or pharmacy chains; refrigerators, ultra-cold refrigerators, or thermal shipper storage; and how quickly vaccination will be used).
The vaccine can be stored for five days at refrigerated 2-8°C conditions.

If using the thermal shipping container for storage, Pfizer protocols and processes include a specific number of minutes the thermal storage shipper can be opened for under various conditions. A timer or timed device that can be easily operated wearing gloves may be helpful for this.
When ready, the vaccines are then thawed, diluted, and used, following Pfizer's detailed protocols. Once thawed and stored under 2-8°C conditions, the
vials cannot be re-frozen or stored under frozen conditions. Further details about how to thaw, prepare, and administer the Pfizer-BioNTech Vaccine, as well as clinical and other administration resources
are available on the CDC Pfizer-BioNTech Vaccine website.
Finally, the thermal shipping container, with the temperature monitor, is returned to Pfizer.
For questions, suggestions, comments, or to share additional COVID-19 training needs, please contact Vanessa Lamers at [email protected].
Additional Pfizer-BioNTech COVID-19 Vaccine Resources for Health Professionals